SAS A00-280 SAS Certified Clinical Trials Programmer Using SAS 9 Exam Practice Test
A Statistical Analysis Plan describes a clinical trial as "A 12 week, double-blind, placebo-controlled, randomized, multi-center study." Double-blind refers to which groups in this study?
Answer : B
Identify the CDISC model with the following characteristics:
* XML-based content and format standard
* facilitates the archive and interchange of the metadata and data for clinical research
* provides an accurate audit trail that is 21 CRF Part II compliant
Answer : B
A subject reports a medication started in March of 2007 but cannot recall the day number. What is the value stored in the SDTM domain CM.CMSTDTC variable?
Answer : D
Which LIBNAME statement is valid?
Answer : D
The VISIT data set is multiple records per subject, sorted by usubjid vistdtc vistm and contains the following variables:

The DEATH data set is one record per subject, sorted by usubjid vistdtc vistm and contains the following variables:
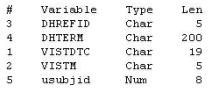
Which program will combine the DEATH and VISIT data sets by matching records?
Answer : A
The following SAS program is submitted:
%let Av=age;
%macro LABD(Av=weight);
%let Av=gend; %mend;
%LABD(Av=height)
%put Av is &Av;
What will be written to the SAS log?
Answer : D
Which clause allows macro variable creation on a select statement in PROC SQL?
Answer : D